Separation of iron filings and sulphur powder compound
Preparation of a mixture and a compound using iron filings and sulphur powder and distinguishing between these
A mixture can be differentiated from a compound in the following manner :
(i) A mixture may be homogeneous or heterogeneous while a compound is always homogeneous.
(ii) Properties of a mixture resemble with the properties of the individual components, whereas the properties of a compound are totally different from constituents from which it is made up of.
(iii) The different components of a mixture can easily be separated by physical means, whereas it is not possible in case of a compound.
(iv) The effect of external factors like heat, light, pressure etc., on a compound and on a mixture of its constituents from which it is made up of, can be different.
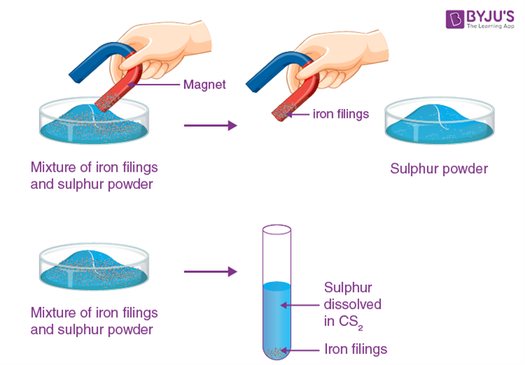
In this experiment, a mixture of iron filing and sulphur represents sample A. It is a mixture whose components can be separated by magnet (when iron filing cling to the magnet) or carbon disulphide (when sulphur dissolves in carbon disulphide).
on heating this mixture strongly, a compound iron (II) sulphide is formed.
A powdered sample of iron (II) sulphide represents Sample B.
Material Required
Iron filings ( 50g),sulphur powder (30g),carbon disulphide,hydrochloric acid,a magnet,Bunsen burner , stand , china dish ,boiling tubes , test tubes,test tube stand ,water glasses , asbestos sheet, pestle and mortar etc.

- Mix 50 g iron filings with 30 g of powdered sulphur throughly. Divide it into two equal parts. Label one part as A. It represents a mixture. Heat the second part in a boiling tube till the contents of the test tube start glowing with a reddish glow. Stop heating. The boiling tube will continue to glow for some time. Cool it throughly by placing it on an asbestos sheet. When the contents of the boiling tube cool, break the boiling tube. Remove the glass pieces. A black mass is obtained. Crush it to a fine powder in a pestle and mortar and label it as sample B. It represents a compound .
- Take the two samples A and B on two watch glasses and label them. Check the two samples for homogeneity or heterogeneity with a naked eye. Record your observations in the note book.
- Take small portions of samples A and B on two separate watch glasses. Move a magnet through each of them and record your observations in the note book.
- Take two test tubes. Put 10 ml of carbon disulphide in each test tube. Add a pinch of A to the first test tube and a pinch of B to the second test tube. Shake vigorously and record your observation in the note book.
- Heat a sample A in a boiling tube. Note down your observations. In a similar manner heat sample B in a boiling tube. Record your observations in the note book.
Observations
Sample A represents a mixture of iron and sulphur while Sample B represents a compound iron (II) sulphide.
- The mixture should be heated strongly to start the reaction.
- Carbon disulphide is highly volatile and its vapour are combustible. It is highly toxic in nature also. Thus, carbon disulphide should be handled with great care.
- To make sure that no iron remains unreacted during the chemical reaction, take a slight excess of sulphur.
- Use an ordinary boiling tube for heating the reaction mixture. Reaction mixture, if heated in a china dish, can catch fire.
Theory
A matter is anything that occupies space and has mass. Based on the composition of the substance, we can further classify matter into two kinds, i.e. mixture and pure substance. We can further divide the mixture into homogenous and heterogeneous mixtures and pure substances into elements and compounds.

Also Read:
- To Prepare a Mixture Using Iron Filings and Sulphur Powder Viva Questions
- To Prepare a Compound Using Iron Filings and Sulphur Powder Viva Questions
What is a mixture?
A mixture is a substance formed by physically mixing two or more substances in any proportion. It doesn’t show any chemical change and retains its constituent property. We can further divide the mixture into homogenous and heterogeneous mixtures. A homogeneous mixture has a uniform composition, while a heterogeneous mixture has a non-uniform composition.
A compound is a substance formed by chemically mixing two or more substances in a fixed proportion. It shows a chemical change and doesn’t retain its constituent property. Any physical method can not separate it.
Difference between Mixture and Compound
| S. No. | Mixture | Compound |
|---|---|---|
| 1. | A mixture is a substance formed by physically mixing two or more substances in any proportion. | A compound is a substance formed by chemically mixing two or more substances in a fixed ratio. |
| 2. | A mixture can either be homogenous or heterogeneous. | The compound is always homogeneous. |
| 3. | The properties of mixtures depend on its constituent. | The properties of compounds don’t rely on its constituent. |
| 4. | Example: Mud water, sugar solution, milk, and blood. | Example: Water, baking soda and salt. |
Mixture

Separation of iron filings and sulphur powder mixture
Compound
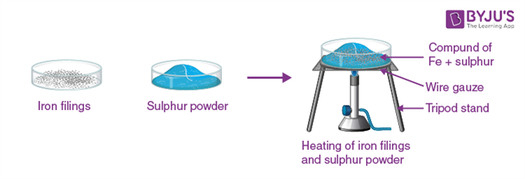
Separation of iron filings and sulphur powder compound