We thank X. Peng (Zhejiang University, China) for valuable advice. We also thank L. Jiang (Institute of Chemistry, Chinese Academy of Sciences) for assistance with the UPS analyses. Portion of the work is carried out at beamline 7.3.3 at the Advanced Light Source, Lawrence Berkeley National Laboratory, which was supported by the US Department of Energy, Office of Science, and Office of Basic Energy Sciences. We acknowledge financial support from the National Natural Science Foundation of China (21975220 and 91833303 (Y.J.); 21922305, 21873080 and 21703202 (L.W.)), Key Research and Development Program of Zhejiang Province (2020C01001 (Y.J.)), Guangdong Major Project of Basic and Applied Basic Research (2019B030302007 (L.Y. and F.H.)), Fundamental Research Funds for the Central Universities (2020XZZX002-06 (L.W.)) and China Postdoctoral Science Foundation (2021M702800 (Y.D.)).
Solution-processed green and blue quantum-dot light-emitting diodes with eliminated charge leakage
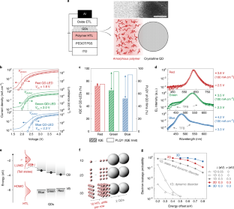
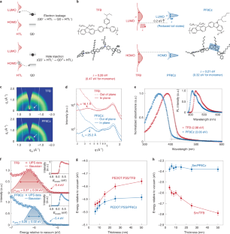
Quantum-dot light-emitting diodes (QD-LEDs) promise a new generation of efficient, low-cost, large-area and flexible electroluminescent devices. However, the inferior performance of green and blue QD-LEDs compared with their red counterpart is hindering the commercialization of QD-LEDs in display and solid-state lighting applications. Here we demonstrate green and blue QD-LEDs with ~100% conversion of the injected charge carriers into emissive excitons. The key to success is the elimination of electron leakage at the organic/inorganic interface by using hole-transport polymers with simultaneous low electron affinity and reduced energetic disorder. Our devices exhibit high external quantum efficiencies over a wide range of luminance values (peak external quantum efficiencies of 28.7% for green and 21.9% for blue) and excellent stability (extrapolated T95 lifetime is 580,000 h for green and 4,400 h for blue QD-LEDs). We expect our work to provide a general strategy for eliminating charge leakage in solution-processed LEDs featuring organic/inorganic interfaces.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
An investigation on the cyclic temperature-dependent performance behaviors of ultrabright air-stable QLEDs
- Saeedeh Mokarian Zanjani
- , Sadra Sadeghi
- … Majid Pahlevani
Scientific Reports Open Access 05 August 2023
Blue light-emitting diodes based on colloidal quantum dots with reduced surface-bulk coupling
- Xingtong Chen
- , Xiongfeng Lin
- … Song Chen
Nature Communications Open Access 17 January 2023
Significant Lifetime Enhancement in QLEDs by Reducing Interfacial Charge Accumulation via Fluorine Incorporation in the ZnO Electron Transport Layer
- Dong Seob Chung
- , Tyler Davidson-Hall
- … Hany Aziz
Nano-Micro Letters Open Access 04 November 2022
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
What is the solution to green plus blue?
The blue-green colour of the solution is due to the formation of copper(II) chloride in the reaction’ this is an example of which kind of chemical reaction?
Open in App
Solution
The blue-green colour solution of copper(II) chloride:
- Copper(II) chloride CuCl 2 gets formed when copper oxide CuO undergoes a reaction with hydrochloric acid HCl .
- The balanced chemical reaction for this is given as follows:
CuO ( s ) Copper oxide + 2 HCl ( aq ) Hydrochloric acid → CuCl 2 ( aq ) Copper ( II ) chloride + H 2 O ( l ) Water
- Copper(II) chloride CuCl 2 is light brown colour solid but, after adsorption of moisture formed during the reaction its colour changes into blue-green.
Hence, the blue-green colour of the solution which is due to the formation of copper(II) chloride in the reaction’ is an example of the reaction of metallic oxide with acid.
Suggest Corrections
Similar questions
Q. On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
(a) Predict the new compound formed which imparts a blue-green colour to solution.
(b) Write a balanced chemical equation of the reaction which takes place.
(c) On the basis of the above reaction, what can you say about the nature of copper oxide?
Q. (a) What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride?
(b) Write the balanced chemical equation for the reaction which takes place.
(c) State the physical conditions of reactants in which the reaction will not take place.
(d) Name the type of chemical reaction which occurs.
(e) Give one example of another reaction which is of the same type as the above reaction.
Q. ‘The blue-green colour of the solution is due to the formation of copper(II) chloride in the reaction’ is an example of which kind of chemical reaction?
Q. A compound X metal oxide is black coloured solid. It reacts with dil. hydrochloric acid and the blue-green coloured solution is formed due to the formation of Y. Identify X and Y and write the chemical reaction involved.
Q. In a chemical laboratory, the teacher takes aqueous solution of silver nitrate ( A g N O 3 ) and adds it to a solution of potassium chloride ( K C l ) . Formation of silver chloride ( A g C l ) , a white solid is observed:
The reaction is an example of a _________.
State the reason of blue-green colour of the solution
Was this answer helpful?
ADVERTISEMENT
Updated on: 21/07/2023
ADVERTISEMENT
The primary colours are red, blue and green.
01:05
View Solution
Give reason Red, blue, and green are called primary colours of light.
02:09
View Solution
ADVERTISEMENT
When an iron nail is immersed in copper sulphate solution, the colour of solution changes from blue to green colour, The substance formed is the solution is_________
02:53
View Solution
______is used for imparting green to blue colour in glasses.
02:45
View Solution
ADVERTISEMENT
Blue green algae contain phycobilin. Which one is blue in colour ?
02:24
View Solution
Blue green algae contain phycobilin. Which one is blue in colour ?
01:55
View Solution
ADVERTISEMENT
Blue colour of blue -green algae is due to
01:54
View Solution
ADVERTISEMENT
State the reason of blue-green colour of the solution
Text Solution
Blue colour of blue -green algae is due to
02:18
On keeping iron nails in a blue coloured copper sulphate solution, it .
01:53
State one reason for adding blue-green algae to the agricultural soil.
02:06
Blue green algae contain phycobilin. Which one is blue in colour ?
01:03
On adding dilute HCl to copper oxide powder, the solution formed is bl.
01:32
State one reason for adding blue-green algae to the agricultural soil.
02:02
Give reasons. (i )Solutions of alkali metals in liquid ammonia are blu.
02:05
Blue green algae contain phycobilin. Which one is blue in colour ?
02:24
- Ask Unlimited Doubts
- Video Solutions in multiple languages (including Hindi)
- Video Lectures by Experts
- Free PDFs (Previous Year Papers, Book Solutions, and many more)
- Attend Special Counselling Seminars for IIT-JEE, NEET and Board Exams
Login
Generate OTP
Notification
Stay updated
Doubtnut wants to send you notifications. Allow to recieve regular updates!
Maybe Later Allow
Listening.
Exams
Online Classes
Previous Year Solutions
- JEE Mains
- JEE Advanced
- NEET – Biology
- NEET – Chemistry
- NEET – Physics
Sample Papers
- NEET Sample Papers
- NEET Biology Sample Papers
- NEET Physics Sample Papers
- NEET Chemistry Sample Papers
- JEE Main Mock Test Papers
- JEE Advanced Mock Test Papers
- Bihar Board Sample Paper
- UP Board Sample Paper
- BITSAT Mock Test Papers
- WBJEE Mock Test Papers
Free Textbook Solutions
- RD Sharma Solutions for Maths
- KC Sinha Solutions for Maths
- Cengage Solutions for Maths
- DC Pandey Solutions for Physics
- HC Verma Solutions for Physics
- Sunil Batra Solutions for Physics
- Pradeep Solutions for Physics
- Errorless Solutions for Physics
- P Bahadur Solutions for Chemistry
- Narendra Awasthi Solutions for Chemistry
- MS Chouhan Solutions for Chemistry
- Errorless Volume 1 Solutions for Biology
- Errorless Volume 2 Solutions for Biology
Free Ncert Solutions English Medium
- NCERT Solutions
- NCERT Solutions for Class 12 English Medium
- NCERT Solutions for Class 11 English Medium
- NCERT Solutions for Class 10 English Medium
- NCERT Solutions for Class 9 English Medium
- NCERT Solutions for Class 8 English Medium
- NCERT Solutions for Class 7 English Medium
- NCERT Solutions for Class 6 English Medium
Free Ncert Solutions Hindi Medium
- NCERT Solutions
- NCERT Solutions for Class 12 Hindi Medium
- NCERT Solutions for Class 11 Hindi Medium
- NCERT Solutions for Class 10 Hindi Medium
- NCERT Solutions for Class 9 Hindi Medium
- NCERT Solutions for Class 8 Hindi Medium
- NCERT Solutions for Class 7 Hindi Medium
- NCERT Solutions for Class 6 Hindi Medium
Boards
Resources
- Unit Converters
- Calculators
- Books Store
- Seminar
- Results
- Exam Corner
- Quiz
- Search Doubtnut
- English Dictionary
- Toppers Talk
- Blogs
- Formula Deck
- Free PDF Solutions
- All in one PDFs
- All in one PDF Class 12
- All in one PDF Class 11
- All in one PDF Class 10
- Class 12 All Books
- Class 11 All Books
- Class 10 All Books
- Class 9 All Books
- Class 8 All Books
- Class 7 All Books
- Class 6 All Books
Doubtnut is No.1 Study App and Learning App with Instant Video Solutions for NCERT Class 6, Class 7, Class 8, Class 9, Class 10, Class 11 and Class 12, IIT JEE prep, NEET preparation and CBSE, UP Board, Bihar Board, Rajasthan Board, MP Board, Telangana Board etc
NCERT solutions for CBSE and other state boards is a key requirement for students. Doubtnut helps with homework, doubts and solutions to all the questions. It has helped students get under AIR 100 in NEET & IIT JEE. Get PDF and video solutions of IIT-JEE Mains & Advanced previous year papers, NEET previous year papers, NCERT books for classes 6 to 12, CBSE, Pathfinder Publications, RD Sharma, RS Aggarwal, Manohar Ray, Cengage books for boards and competitive exams.
Doubtnut is the perfect NEET and IIT JEE preparation App. Get solutions for NEET and IIT JEE previous years papers, along with chapter wise NEET MCQ solutions. Get all the study material in Hindi medium and English medium for IIT JEE and NEET preparation